Abstract
Pontodrilus litoralis is a cosmopolitan littoral earthworm known to exhibit bioluminescence. Recently, a congeneric species, Pontodrilus longissimus, from Thailand was described. These species are sympatric, but their burrowing depths on Thai beaches are different. In this study, we examined the in vivo and in vitro bioluminescent properties of P. longissimus and P. litoralis. Mechanical stimulation induced in vivo luminescence in P. litoralis, as reported previously, but not in P. longissimus. In vitro cross-reaction tests between these species revealed the absence of luciferin and luciferase activities in P. longissimus. The coelomic fluid of P. litoralis had strong fluorescence that matched the spectral maximum of its bioluminescence, but the same result was not observed for P. longissimus. These results suggest that P. litoralis has luminescence abilities due to the creation of bioluminescent components (i.e., luciferin, luciferase, and light emitters). The presence of both luminous and nonluminous species in a single genus is likely widespread, but only a few examples have been confirmed. Our findings provide insight into the possible functions of bioluminescence in earthworms, such as avoiding predation by littoral earwigs.
Similar content being viewed by others
Introduction
The earthworm genus Pontodrilus Perrier, 1874, displays various unique characteristics. The littoral earthworm P. litoralis (Grube, 1855) is distributed on the tropical and subtropical coasts of the Atlantic, Indian and Pacific Oceans1,2,3 and is known to be bioluminescent4,5,6,7. The luminescent system of P. litoralis has been shown to be a luciferin-luciferase type reaction triggered by hydrogen peroxide, with a fluorescence compound acting as a light emitter7, although the chemical structure of the luciferin remains uncertain and the luciferase gene has not been determined. Recently, the littoral earthworm Pontodrilus longissimus Seesamut & Panha, 2018 was identified in coastal areas in Thailand and peninsular Malaysia8 and determined to be a separate species based on morphological differences from other congeners in the size of the body, the number of segments and the diverticulum.
In the present study, the bioluminescence and fluorescence of P. litoralis and P. longissimus were examined in vivo and in vitro, and the results suggested that P. longissimus lacks luminescence ability despite its genetically close relationship to P. litoralis. Based on these findings, we discuss the biological function of earthworm bioluminescence and a convenient parataxonomic method for Pontodrilus species.
Results
In vivo and in vitro bioluminescence
After the live specimens of both Pontodrilus species were stimulated by electricity or rough handling, P. litoralis exuded a green luminescent fluid, whereas the fluid exuded from P. longissimus was not luminescent (Fig. 1A). Under a handheld longwave UV lamp (365 nm), almost the entire body of P. litoralis emitted strong yellow fluorescence, which was most conspicuous in the rows of setae, whereas P. longissimus did not emit fluorescence under the same conditions (Fig. 1B).
The cross-reactivities of crude luciferase and crude luciferin in P. litoralis and P. longissimus were examined (Fig. 2A). To ensure the validity of the results, we used a concentration of P. longissimus extract that was fivefold higher than the concentration of P. litoralis extract. The results showed that significant luminescence was detected only when mixing the luciferin extract from P. litoralis with the luciferase extract from P. litoralis. On the other hand, both luciferin and luciferase activities in the extracts of P. longissimus were negative (the levels of both activities were almost the same as those in a negative control).
(A) Cross-reaction between crude luciferase and luciferin of the littoral earthworm genus Pontodrilus. Plit represents P. litoralis, Plon represents P. longissimus, and Buff represents the buffer (50 mM Tris–HCl, pH 7.2). Data are shown as the mean ± standard deviation (n = 3). (B) Fluorescence spectra of crude coelomic fluid extracted from P. litoralis. An excitation spectrum (solid blue line) with emission at 523 nm, an emission spectrum (dashed red line) with excitation at 453 nm, and an emission spectrum (solid red line) with excitation at 370 nm were obtained. The dashed black line shows the in vivo luminescence spectrum of live P. litoralis. The solid black line shows the in vitro luminescence spectrum of crude coelomic fluid extracted from P. litoralis, which contained 500 µl 50 mM Tris–HCl at pH 7.2 and 50 µl 0.3% hydrogen peroxide. The data shown are the means of five measurements.
Fluorescence and luminescence spectra of luminous P. litoralis
Fluorescence spectra were measured using a crude coelomic fluid extracted from P. litoralis (Fig. 2B). The peaks of the excitation spectra were 370 and 453 nm, whereas the emission peaks were 450 and 523 nm, indicating the presence of at least two fluorescent compounds in P. litoralis; however, the possibility of the presence of a single dual-chromophore molecule, such as a genetically designed biosensor, cannot be excluded. The luminescence spectra of P. litoralis had maximum wavelengths of 528 nm in vivo and 524 nm in vitro (Fig. 2B). Wampler and Jamieson showed that the spectral maximum of bioluminescence (540 nm) in Bermudan P. bermudensis (which is now considered synonymous with P. litoralis2) matched the fluorescence maximum of the coelomic fluid and suggested that the fluorescent substance is a light emitter7. Although the maximum spectral values in our study were different from those in their results, probably due to genetic differences in the specimens examined or differences in the spectrophotometers used, our results also showed a spectral match between fluorescence and bioluminescence in vitro. The small red shift in the in vivo spectrum might have been due to a reflection effect from the reddish earthworm body.
Comparison of the coelomic fluid cells and protein bands between the two littoral earthworm species
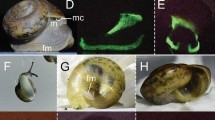
The coelomic cells of the littoral earthworm species were observed under a fluorescence microscope (Fig. 3). The results showed that the coelomic cells of P. litoralis emitted fluorescence, while those of P. longissimus did not. The diameter of the coelomic cells was approximately 15 µm, and numerous small fluorescent particles were detected in the coelomic cells of P. litoralis. SDS-PAGE of the coelomic fluids showed different protein constitutions between the two species (Supplementary Fig. S1).
(A) Bright field image of P. litoralis coelomic cells (arrowheads). (B) Bright field image of P. longissimus coelomic cells. (C) P. litoralis coelomic cells under UV irradiation at 380 nm. (D) P. longissimus coelomic cells under UV irradiation at 380 nm. The photography conditions in (C) were the same as those in (D), but no fluorescence was observed in the latter image.
Discussion
In this study, we confirmed that P. longissimus is nonbioluminescent, despite its close relationship to the luminous species P. litoralis (Supplementary Fig. S2)8. The presence of both luminous and nonluminous species in a single genus is likely widespread, but only a few examples have been confirmed; for example, the genera Vibrio and Photobacterium (marine bacteria)9, Epigonus (deep-sea fishes)10, Mycena (bonnet mushrooms)11 and Eisenia (terrestrial earthworms)12 have been reported to contain both luminous and nonluminous species. P. litoralis and P. longissimus can easily be collected at the same beach8 and reared in a laboratory; thus, they are suitable for studying the ecology and evolution of bioluminescence.
In vitro luciferin-luciferase cross-reaction tests of P. longissimus and P. litoralis confirmed that the luminescence ability of P. litoralis is due to the presence of multiple bioluminescent components in coelomic fluid, i.e., luciferin, luciferase and the light emitter. Cross-reaction tests have previously indicated that luminous earthworms in the genera Pontodrilus (Megascolecidae), Microscolex and Diplocardia (Acanthodrilidae) share the same basic bioluminescence mechanisms5,7,13,14, despite their distant relationships to each other15,16. It is expected that the ancestral state of Pontodrilus is nonbioluminescent because the nearest extant relatives of Pontodrilus belong to the genus Plutellus Perrier, 1873, and all members of this group are nonbioluminescent6,17. These findings suggested that P. litoralis secondarily acquired bioluminescent properties through parallel evolution, similar to the case of bioluminescence in lampyrid and elaterid beetles18. We detected a clear difference in the protein composition of the secreted fluid between P. litoralis and P. longissimus (Supplementary Fig. S1). Luciferase and other bioluminescent components of luminous earthworms were not identified, and further comparative analyses of the protein bands, which appear only in the secreted fluid of luminous species, will be useful to understand the mechanisms of bioluminescence and its parallel evolution.
In Thailand, P. longissimus was found sympatrically with P. litoralis at the beaches along the coast, but the microhabitats of the two congeners are different; P. litoralis was collected on the beach surface (under trash or leaf litter on sandy beaches), whereas P. longissimus was found at a greater depth than P. litoralis, i.e., a depth of more than 10 cm, where trash and leaves are scarce8 (Fig. 4A–D). It has been hypothesized that the biological function of bioluminescence in Annelida, including P. litoralis, is to stun or divert attention as an anti-predator defense19,20,21,22,23,24,25, but experiments and observations of the prey are limited. Sivinski & Forrest25 reported that the luminescence of Microscolex phosphoreus deterred predation by the mole cricket Scapteriscus acletus under laboratory conditions, although the specimen was ultimately consumed. A British television program26 presented by David Attenborough showed that the French luminous earthworm Avelona ligra glowed when attacked by the carabid beetle, but the beetle consumed the luminescent worm without any hesitation. We suggest that the absence of bioluminescence in P. longissimus may be associated with its presence in habitats with low predation pressure, whereas P. litoralis acquired a bioluminescent property during evolution that enabled it live on the surface of the beach, which is rich in nutrition and food sources3,27 as well as potential predators.
(A) The microhabitat of Pontodrilus litoralis from Aichi Prefecture, Japan. (B) The microhabitat of P. longissimus in Ranong, Thailand; sympatric Pontodrilus specimens were collected from this location8. (C) P. longissimus was found at a depth of 10–30 cm in muddy sand; the earthworm is shown by an arrow. (D) Bright field image of the Pontodrilus species included in this study. (E) An earwig (Anisolabis maritima) (a potential Pontodrilus predator) grooming its forelegs after attacking P. litoralis. (F) A. maritima (arrowhead) was found in the same microhabitat as P. litoralis in Aichi Prefecture, Japan.
Indeed, while we observed some burrowing bivalves, no potential predators were observed in the deep sand inhabited by P. longissimus. In contrast, various carnivorous invertebrates, such as earwigs, rove beetles and carabid beetles, were observed on the surface of beaches in Thailand and Japan, where P. litoralis live (Seesamut pers. obs.). We therefore performed a feeding experiment using maritime earwigs sympatrically distributed in a P. litoralis habitat. The maritime earwig Anisolabis maritima (Dermaptera, Anisolabididae) is a cosmopolitan species that can be found in Japan. It has well-developed compound eyes (Fig. 4E) and is considered a carnivorous animal that forages for prey at night28, 29. A. maritima (body length ≤ 30 mm) was the predominant predator at the beach where P. litoralis was collected (Fig. 4F). Some rove beetles (Coleoptera, Staphylinidae) were found in the same habitat, but they seemed to be too small (< 10 mm) to consume P. litoralis, and during our laboratory observations, the rove beetle did not attack the worm. Thus, we think A. maritima is a major potential predator of P. litoralis at the beaches in Japan. Live P. litoralis and A. maritima were collected from the same beach on the same day, and we observed the predation behavior by the latter in a laboratory within a dark cage with beach sand spread on the bottom. Our observation of the predation of P. litoralis by earwigs (Supplementary Video 1) may provide important insight into the function of bioluminescence in P. litoralis. The earwigs immediately began aggressively attacking the worm with their mandibles and abdominal cerci, a pair of scissor-like pincers; the worm secreted luminescent mucus from its wounds (Supplementary Video 1), and it appeared that the retention of the glue-like luminescent mucus on the mouth and forelegs of the earwigs was unpleasant to them, since they attempted to remove the mucus by frequent grooming (Fig. 4E, Supplementary Video 2). Indeed, after aggressive attacks, the earwigs ultimately abandoned their attempts to consume the worm, and thus, the worm survived. To the best of our knowledge, this is the first observation of earthworm bioluminescence induced by predation under almost natural conditions. Based on these observations, we hypothesized that the luminous glue-like mucus of P. litoralis may function to deter and/or divert predation and that luminescence might even enhance the avoidance learning of the predator. Notably, the amount of mucus exuded following the same mechanical stimulation seemed to be much higher in P. litoralis than in P. longissimus. Nevertheless, we suppose that the global distribution of P. litoralis is a consequence of its adaptation to the beach surface (i.e., luminescence), which provides opportunities for dispersal by current, whereas P. longissimus is endemic to the coasts of the Thai-Malay peninsula8,30 due to its inhabitation of deeper sand.
Based on microscopic observations, we confirmed that both species secrete coelomic cells following stimulation, but neither bioluminescence nor fluorescence was observed in P. longissimus. The presence and absence of fluorescence in a single genus of earthworm was also reported in the terrestrial genus Eisenia, and it has been suggested that the difference in fluorescent characteristics could be used as a “fluorescence fingerprint” to delineate these closely related species31. Therefore, the fluorescence fingerprint method is also applicable to Pontodrilus.
Littoral zones have rich species diversity of both macro- and microorganisms32,33. They comprise a front of human pressure in marine ecology and are one of the most important zones for conservation34,35; therefore, there is a need to understand littoral fauna. Earthworms typically have strong effects on soil ecosystems36,37,38. Pontodrilus is a major “ecosystem engineer”38 that inhabits littoral habitats. Thus, the identification of P. litoralis and P. longissimus is significant to the assessment of the littoral environment. These species are actually distinguishable by the internal morphology of the spermathecal diverticulum, but special skills and equipment are necessary for morphological analyses. In this study, we identified differences in the bioluminescent and fluorescent characteristics of P. litoralis and P. longissimus and demonstrated that the analysis of these differences provides an easy in situ methodology to identify these earthworms in marine ecological studies and for the conservation of littoral zones in Southeast Asia.
Methods
Specimens and species identification
The littoral earthworm P. litoralis was collected at a sandy region of one of the following beaches3,8: Wonnapa Beach, Amphoe Mueang Chon Buri, Chonburi, Thailand (13°15′55.6"N 100°55′29.3"E); Kowa Beach, Chita, Aichi Prefecture, Japan (34°46′23.3"N 136°54′52.7"E); and Kira Waikiki Beach, Nishio, Aichi Prefecture, Japan (34°46′55.2"N 137°05′48.3"E). P. longissimus was collected from Tambon Muang Klang, Amphoe Kaper, Ranong, Thailand (9°37′26.7"N 98°28′08.6"E). The earthworms were maintained in native sand in plastic containers sprayed with artificial seawater to keep the sand moist. Species identification was performed based on morphological characteristics by Seesamut et al.8. In vivo bioluminescence was photographed in darkness with a Nikon D5500 digital camera (Nikon, Japan). Pontodrilus were stimulated by electricity or rough handling to induce bioluminescence, and in vivo fluorescence was photographed under a handheld UV lamp (365 nm) without mechanical stimulation.
Extraction of the luminescent substance
To prepare crude Pontodrilus luciferase and luciferin, live earthworms were rinsed with distilled water and transferred for 24 h to Petri dishes with wet tissue paper moistened with artificial seawater to avoid contamination by their stomach contents when extracting coelomic fluid. All experiments were carried out on ice except for the measurements of light intensity and spectra. Coelomic fluid was extracted as follows: twenty live worms of each species (2.72 g wet weight of P. litoralis and 7.4 g wet weight of P. longissimus) were put on a mortar and stimulated with a pestle to induce exudation of coelomic fluid, and then 10 ml of 50 mM Tris–HCl at pH 7.2 was added. After removing the specimens, the solution was centrifuged at 15,000 × g for 15 min at 4 °C in a TOMY MX-100 high speed refrigerated microcentrifuge (Tomy Seiko, Japan), and the supernatants were collected as the crude extracts. The crude luciferin and luciferase fractions were prepared based on the method described by Bellisario et al.39. In brief, the crude extracts were filtered using a 10 K centrifugal filter device (Merck, Germany); the first flow through was used as a crude luciferin extract, and the retentates on the membranes were collected as crude luciferase extract.
Cross-reaction experiment and spectral measurement
The total protein concentrations of the crude luciferase extracts measured using a protein assay kit (Bio-Rad, USA) were 19.56 µg/ml in P. litoralis and 102.78 µg/ml in P. longissimus. The luminescent activity was monitored using a luminometer (Centro LB960, Berthold). Ten microliters of crude luciferase was mixed with 40 µl of crude luciferin, and 10 µl of 0.3% hydrogen peroxide was injected to initiate the luminescence reaction. The luminescence was recorded in relative light units (RLUs) for a total of 120 s after hydrogen peroxide injection. Three replicate measurements were performed.
Spectral measurement
Luminescence and fluorescence spectra were recorded with a spectrofluorometer (JASCO, FP-777 W). For the luminescence spectra measurements, the excitation light source was shut off. The data were smoothed using a binomial method, and the spectral response was not corrected. An in vivo luminescence spectrum was obtained using a single living specimen put into a quartz cuvette immediately after stimulation by rough handling. To obtain an in vitro luminescence spectrum, 100 µl of crude luciferase and 300 µl of luciferin were mixed with 400 ml of 50 mM Tris–HCl at pH 7.2 and 40 µl of 0.3% hydrogen peroxide, and luminescence was immediately measured. Fluorescence spectra of coelomic fluid from P. litoralis were obtained using crude extract suspended in 500 µl of 50 mM Tris–HCl at pH 7.2. The bandwidths used for emission and excitation were 5 nm.
Coelomic cell photography and SDS-PAGE
Coelomic cells of Pontodrilus were isolated by stimulating earthworms on microscope slides, observed under a fluorescence microscope (Nikon Eclipse E600, Japan) with a 60 × objective lens (Nikon CFI Plan Fluor Series, Japan), and photographed using an attached digital camera (Nikon D5500, Japan). The fluorescence excitation was performed at 380 nm.
The crude coelomic extract of both species was run on a 15% SDS-PAGE gel using a 1D Gel Electrophoresis Mini Gel, AE-6530mPAGE (ATTO), followed by silver staining (Silver Stain MS Kit, FUJIFILM Wako Pure Chemical Corporation).
Video recording
Video recording of the live specimens was performed using a Nikon D500 and Micro NIKKOR 60 mm lens (Nikon) under red light (LED Lenser T2QC) with the following settings: ISO, 64000; f/2.8; and exposure, 1/60 s.
References
Gates, G. E. Burmese earthworms, an introduction to the systematics and biology of megadrile oligochaetes with special reference to South-east Asia. Trans. Am. Philos. Soc. 62, 1–326. https://doi.org/10.2307/1006214 (1972).
Easton, E. G. Earthworms (Oligochaeta) from islands of the south-western Pacific, and a note on two species from Papua New Guinea. N. Z. J. Zool. 11, 111–128. https://doi.org/10.1080/03014223.1984.10423750 (1984).
Seesamut, T., Jirapatrasilp, P., Chanabun, R., Oba, Y. & Panha, S. Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan. Zookeys. 862, 23–43. https://doi.org/10.3897/zookeys.862.35727 (2019).
Kanda, S. The luminescence of Pontodrilus matsushimensis. Rigakukai 36, 1–7 (1938).
Jamieson, B. G. M. & Wampler, J. E. Bioluminescent Australian Earthworms II. Taxonomy and preliminary report of bioluminescence in the general Spenceriella, Fletcherodrilus and Pontodrilus (Megascolecidae: Oligochaeta). Aust. J. Zool. 27, 637–669. https://doi.org/10.1071/ZO9790637 (1979).
Wampler, J. E. & Jamieson, B. G. M. Earthworm bioluminescence: comparative physiology and biochemistry. Comp. Biochem. Physiol. 66, 43–50. https://doi.org/10.1016/0305-0491(80)90081-4 (1980).
Wampler, J. E. & Jamieson, B. G. M. Cell bound bioluminescence from Pontodrilus bermudensis, and its similarities to other earthworm bioluminescence. Comp. Biochem. Physiol. 84, 81–87. https://doi.org/10.1016/0300-9629(86)90046-0 (1986).
Seesamut, T., Sutcharit, C., Jirapatrasilp, P., Chanabun, R. & Panha, S. Morphological and molecular evidence reveal a new species of the earthworm genus Pontodrilus Perrier, 1874 (Clitellata, Megascolecidae) from Thailand and Peninsular Malaysia. Zootaxa 4496, 218–237. https://doi.org/10.11646/zootaxa.4496.1.18 (2018).
Dunlap, P. V. & Urbanczyk, H. Luminous Bacteria. In The Prokaryotes: Prokaryotic Physiology and Biochemistry (eds Rosenberg, E. et al.) 495–528 (Springer, 2013).
Okamoto, M. & Gon, O. A review of the deepwater cardinalfish genus Epigonus (Perciformes: Epigonidae) of the Western Indian Ocean, with description of two new species. Zootaxa 4382, 261–291. https://doi.org/10.11646/zootaxa.4382.2.3 (2018).
Ke, H. M. et al. Mycena genomes resolve the evolution of fungal bioluminescence. PNAS 117, 31267–31277. https://doi.org/10.1073/pnas.2010761117 (2020).
Pes, O., Midlik, A., Schlaghamersky, J., Zitnan, M. & Taborsky, P. A study on bioluminescence and photoluminescence in the earthworm Eisenia lucens. Photochem. Photobiol. Sci. 15, 175–180. https://doi.org/10.1039/C5PP00412H (2016).
Wampler, J. E. The bioluminescence system of Microscolex phosphoreus and its similarities to those of other bioluminescent earthworms (Oligochaeta). Comp. Biochem. Physiol. 71, 599–604. https://doi.org/10.1016/0300-9629(82)90209-2 (1982).
Rodionova, N. S. & Petushkov, V. N. Comparison of Earthworm Bioluminescent Systems. Dokl. Biochem. Biophys. 485, 157–161. https://doi.org/10.1134/S1607672919020224 (2019).
James, S. W. & Davidson, S. K. Molecular phylogeny of earthworms (Annelida: Crassiclitellata) based on 28S, 18S and 16S gene sequences. Invertebr. Syst. 26, 213–229. https://doi.org/10.1071/IS11012 (2012).
Jamieson, B. et al. Phylogeny of the Megascolecidae and Crassiclitellata (Annelida, Oligochaeta): combined versus partitioned analysis using nuclear (28S) and mitochondrial (12S, 16S) rDNA. Zoosystema 24, 707–734 (2002).
Blakemore, R. J. Origin and means of dispersal of cosmopolitan Pontodrilus litoralis (Oligochaeta: Megascolecidae). Eur. J. Soil Biol. 43, S3–S8. https://doi.org/10.1016/j.ejsobi.2007.08.041 (2007).
Fallon, T. R. et al. Firefly genomes illuminate parallel origins of bioluminescence in beetles. eLife 7, e36495. https://doi.org/10.7554/eLife.36495 (2018).
Gouveneaux, A. & Mallefet, J. Physiological control of bioluminescence in a deep-sea planktonic worm, Tomopteris helgolandica. J. Exp. Biol. 216, 4285–4289. https://doi.org/10.1242/jeb.090852 (2013).
Haddock, S. H. D., Moline, M. A. & Case, J. F. Bioluminescence in the sea. Annu. Rev. Mar. Sci. 2, 443–493. https://doi.org/10.1146/annurev-marine-120308-081028 (2010).
Huber, M. E., Arneson, C. A. & Widder, E. A. Extremely blue bioluminescence in the polychaete Polycirrus perplexus (Terebellidae). Bull. Mar. Sci. 44, 1236–1239 (1989).
Oba, Y. & Schultz, D. T. Eco-Evo bioluminescence on land and in the sea. In Bioluminescence: Fundamentals and Applications in Biotechnology Vol. 1 (eds Thouand, G. & Marks, R.) 3–36 (Springer, 2014).
Verdes, A. & Gruber, D. F. Glowing worms: biological, chemical, and functional diversity of bioluminescent annelids. Integr. Comp. Biol. 57, 18–32. https://doi.org/10.1093/icb/icx017 (2017).
Shimomura, O. & Yampolsky, I. Bioluminescence: Chemical Principles and Methods 3rd edn. (World Scientific, 2019).
Sivinski, J. & Forrest, T. Luminous defense in an earthworm. Fla. Entomol. 66, 517 (1983).
Attenborough, D. Life that grows. BBC Television, 9 May 2016.
Coupland, G. T. & McDonald, J. I. Extraordinarily high earthworm abundance in deposits of marine macrodetritus along two semi-arid beaches. Mar. Ecol. Prog. Ser. 361, 181–189. https://doi.org/10.3354/meps07351 (2008).
Guppy, R. Biology of Anisolabis maritima (Gene) the seaside earwig, on Vancouver Island (Dermaptera, Labiduridae). Proc. Entomol. Soc. Brit. Col. 16, 14–18 (1950).
Langston, R. L. The maritime earwig in California (Dermaptera: Carcinophoridae). Pan Pac. Entomol. 50, 28–34 (1974).
Seesamut, T., Jirapatrasilp, P., Sutcharit, C., Tongkerd, P. & Panha, S. Mitochondrial genetic population structure and variation of the littoral earthworm Pontodrilus longissimus Seesamut and Panha, 2018 along the coast of Thailand. Eur. J. Soil Biol. 93, 103091. https://doi.org/10.1016/j.ejsobi.2019.103091 (2018).
Albani, J. R., Demuynck, S., Grumiaux, F. & Leprêtre, A. Fluorescence fingerprints of Eisenia fetida and Eisenia andrei. Photochem. Photobiol. 78, 599–602. https://doi.org/10.1562/0031-8655(2003)078%3c0599:FFOEFA%3e2.0.CO;2 (2003).
Satyam, K. & Thiruchitrambalam, G. Habitat Ecology and Diversity of Rocky Shore Fauna. In Biodiversity and Climate Change Adaptation in Tropical Islands (eds Sivaperuman, C. et al.) 187–215 (Elsevier, London, 2018). https://doi.org/10.1016/B978-0-12-813064-3.00007-7.
Zohary, T. & Gasith, A. The Littoral Zone. In Lake Kinneret: Ecology and Management, (eds. Zohary, T., Sukenik, A., Berman, T. & Nishri, A.) 517–532 (Springer, Dordrecht, 2014). http://dx.doi.org/https://doi.org/10.1007/978-94-017-8944-8_29
Banks, S. A., Skilleter, G. A. & Possingham, H. P. Intertidal habitat conservation: identifying conservation targets in the absence of detailed biological information. Aquatic Conserv Mar. Freshw. Ecosyst. 15, 271–288. https://doi.org/10.1002/aqc.683 (2005).
Butler, R. G. & de Maynadier, P. G. The significance of littoral and shoreline habitat integrity to the conservation of lacustrine damselflies (Odonata). J. Insect Conserv. 12, 23–36. https://doi.org/10.1007/s10841-006-9059-0 (2008).
González, G., Seastedt, T. R. & Donato, Z. Earthworms, arthropods and plant litter decomposition in aspen (Populus tremuloides) and lodgepole pine (Pinus contorta) forests in Colorado, USA. Pedobiologia 47, 863–869. https://doi.org/10.1078/0031-4056-00272 (2003).
Kooch, Y. & Jalilvand, H. Earthworms as ecosystem engineers and the most important detritivors in forest soils. Pak. J. Biol. Sci. 11, 819–825. https://doi.org/10.3923/PJBS.2008.819.825 (2008).
Lavelle, P. et al. Soil function in a changing world: the role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 33, 159–193 (1997).
Bellisario, R., Spencer, T. E. & Cormier, M. J. Isolation and properties of luciferase, a non-heme peroxidase, from the bioluminescent earthworm, Diplocardia longa. Biochemistry 11, 2256–2266. https://doi.org/10.1021/bi00762a008 (1972).
Acknowledgements
We thank Gaku Mizuno (Chubu University) and members of the Animal Systematics Research Unit, Chulalongkorn University, for assistance in collecting materials. This work was partly supported by JST CREST (JPMJCR16N1) to Y.O.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.S. and Y.O.; Fieldwork, T.S., I.K. and S.P.; methodology, T.S., D.Y., J.P. and I.K.; investigation, T.S., D.Y., J.P. and Y.O.; writing original draft preparation, T.S.; writing, review and editing, T.S.; Y.O.; supervision, S.P. and Y.O. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seesamut, T., Yano, D., Paitio, J. et al. Occurrence of bioluminescent and nonbioluminescent species in the littoral earthworm genus Pontodrilus. Sci Rep 11, 8407 (2021). https://doi.org/10.1038/s41598-021-87984-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87984-4
This article is cited by
-
Global species delimitation of the cosmopolitan marine littoral earthworm Pontodrilus litoralis (Grube, 1855)
Scientific Reports (2024)
-
Responses to salinity in the littoral earthworm genus Pontodrilus
Scientific Reports (2022)
-
Bioluminescence in aquatic and terrestrial organisms elicited through various kinds of stimulation
Aquatic Ecology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.